Malaria remains a daily threat across Africa. In 2023 alone, the continent accounted for 95% of the world’s 597,000 malaria-related deaths. The most vulnerable are our children and pregnant women. Every minute, a child in Africa dies from this disease.
But malaria doesn’t just rob us of lives, it also drains our economies. Across Africa, the disease costs us an estimated $12 billion each year. In Uganda, my home country, we lose more than $500 million per decade. During peak seasons, malaria can cause up to half of all outpatient visits and nearly a fifth of hospital admissions.
Worse still, the challenges are growing. Climate change, resistance to insecticides, and reduced effectiveness of antimalarial drugs are threatening the progress we’ve made. So, we’re left with a difficult question: Must we accept malaria as an inevitable part of life?
I don’t believe we should. And as both a scientist and a mother, I believe we can do more.
At Target Malaria, the nonprofit research consortium I work with, we’re exploring a new frontier in the fight against malaria: gene drive technology.
Here’s how it works: We introduce a genetic trait into the Anopheles mosquito, one of the main carriers of the malaria parasite. This gene is passed down to offspring at a higher-than-normal rate, gradually reducing the population’s ability to reproduce. Over time, the mosquito population declines — potentially enough to interrupt malaria transmission altogether.
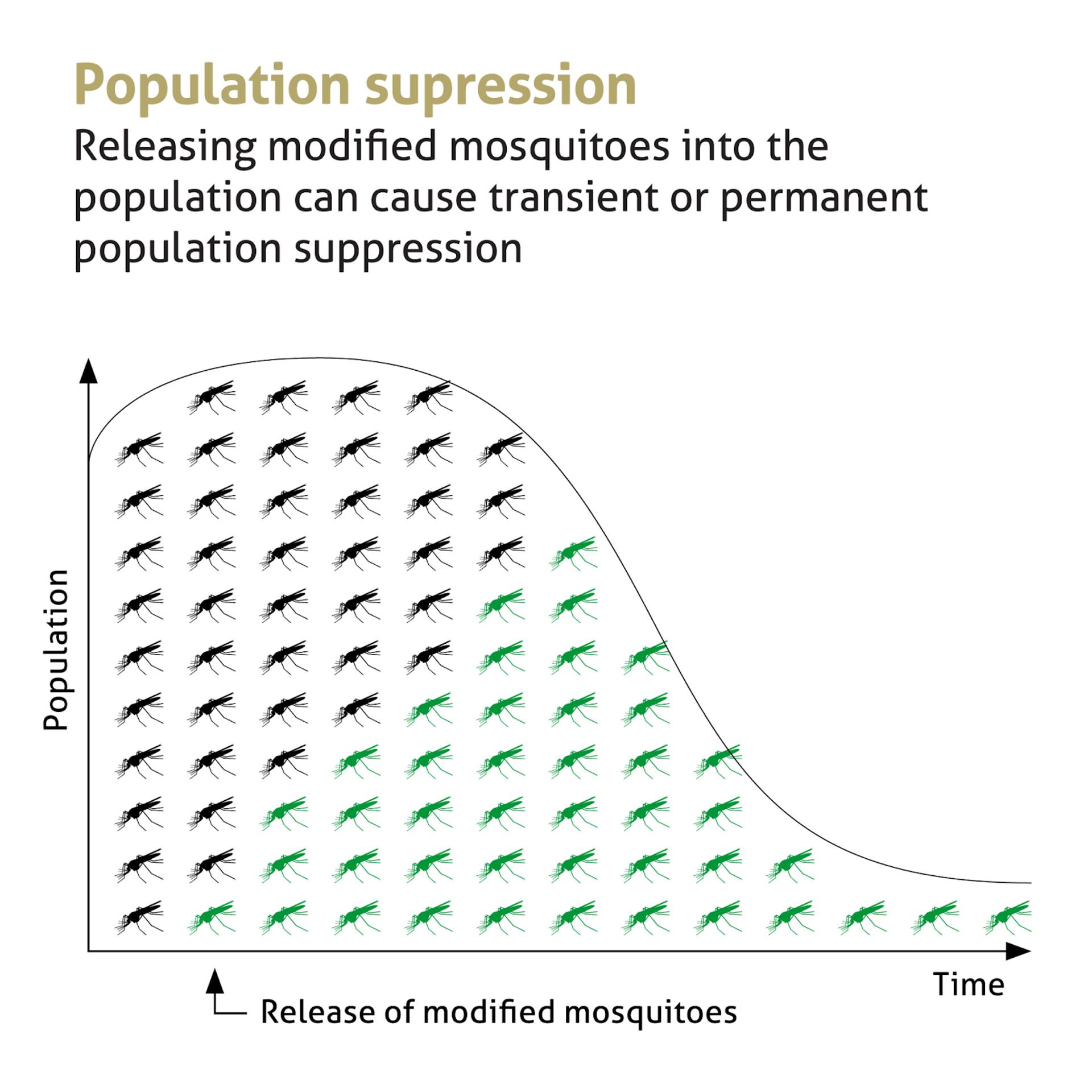
A large-scale modeling study across 13 West African countries showed that gene drive mosquitoes could reduce populations of malaria-transmitting species by 71% to 98%. When combined with existing tools like vaccines and new-generation mosquito nets, it could prevent up to 60% more clinical malaria cases.
This technology is not designed to eradicate all mosquitoes — nor could it. Of more than 3,500 known mosquito species, only about 30 are a public health concern. Of those, just three are responsible for most malaria transmission in Africa. Our work is focused on this narrow target.
Genetic approaches also offer some clear advantages for Africa’s unique health landscape. They require less day-to-day management than current interventions, making them particularly suited for rural and hard-to-reach communities. And because they are species-specific, they offer a more environmentally targeted solution. But we are taking a cautious, transparent approach. We’re still years away from releasing gene drive mosquitoes into the wild — and we won’t take that step without the required regulatory approvals and the agreement of the communities involved.
In the meantime, we are conducting rigorous ecological studies to ensure that reducing one mosquito species won’t cause ripple effects in the environment. Early results of ecological studies conducted on the Anopheles mosquitoes in Ghana are promising. Animals, like birds and bats, that prey on these mosquitoes appear to have a varied diet and thus would be able to survive without them.
We’re also listening to community members, civil society groups, and environmental advocates have voiced concerns because science must serve people, not just data.
Malaria isn’t just a scientific challenge — it’s personal. I have lost people I love to this disease. I have watched it strike hardest in communities that already face poverty, limited health access, and climate vulnerability. Science gives us the tools to change the future.
Gene drive won’t be a silver bullet. But as part of an integrated approach alongside existing tools and partnerships, it could help turn one of our oldest enemies into an unlikely ally.
This isn’t science fiction. It’s science — African-led, community-centered, and driven by hope.
And that’s a future worth fighting for.
Krystal Birungi is an entomologist at the Uganda Virus Research Institute, and a Research and Outreach Associate at Target Malaria.

